Hoyer Laboratory
Neuronal Organelle Quality Control and Health
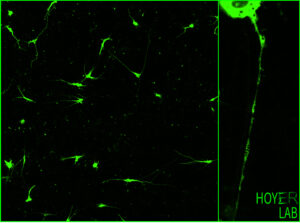 Eliminating specific protein targets or even entire cellular organelles is important for generating and maintaining healthy neurons. The Hoyer Lab aims to understand how neurodegenerative diseases can be prevented by improving these key quality control mechanisms. Our target organelle is the endoplasmic reticulum (ER), which is essential to neuronal connectivity. The ER network has many important functions such as providing a site for protein synthesis, storing and releasing calcium for signaling, and forming regulatory membrane contact sites with other organelles. Importantly, impaired ER network formation or defective ER clearance mechanisms are linked to neurological disorders.
Eliminating specific protein targets or even entire cellular organelles is important for generating and maintaining healthy neurons. The Hoyer Lab aims to understand how neurodegenerative diseases can be prevented by improving these key quality control mechanisms. Our target organelle is the endoplasmic reticulum (ER), which is essential to neuronal connectivity. The ER network has many important functions such as providing a site for protein synthesis, storing and releasing calcium for signaling, and forming regulatory membrane contact sites with other organelles. Importantly, impaired ER network formation or defective ER clearance mechanisms are linked to neurological disorders.
The Hoyer Lab characterizes ER surveillance mechanisms at the molecular level by combining genetic manipulation of stem cells, which can be efficiently converted to different neuronal cell populations. The lab employs various investigative approaches including quantitative proteomics, live cell fluorescence microscopy, high resolution cryo-electron tomography and other biochemical techniques.
Our research covers three main areas with the goal of understanding ER quality control and function in neurons:
- Characterize upstream signals, molecular machinery, and spatio-temporal properties of ER removal through selective autophagy (“ER-phagy”) or other ER protein clearance mechanisms.
- Study the consequences of disrupted ER proteins levels in different types of neuronal cell types.
- Analyze ER proteins directly related to neurological disorders by performing systematic genetic modifications and quantitative proteomics.
News & Featured Publications
Learn More
The building blocks of brain cells

Van Andel Institute announces 2025 Public Lecture Series

Van Andel Institute recruits neuronal cell biology expert Dr. Melissa Hoyer
Hoyer MJ, Smith IR, Paoli, JC, Jiang, Y, Paulo JA, Harper JW. 2024. Combinatorial selective ER-phagy remodels the ER during neurogenesis. Nat Cell Biol 26:378–392.
*Featured on the cover
Eapen V*, Swarup S*, Hoyer MJ*, Paulo J, Harper JW. 2021. Quantitative proteomics reveals the selectivity of ubiquitin-binding autophagy receptors in the turnover of damaged lysosomes by lysophagy. eLife 10:e72328.
Hoyer MJ, Chitwood PJ, Ebmeier, CC, Striepen, JF, Qi, RZ, Old WM, Voeltz GK. 2018. A novel class of ER membrane proteins regulates ER-associated endosome fission. Cell 175: 254–265.e14.
Our Impact
We're raising thousands to save millions
We’re turning hope into action for the millions of people around the world affected by diseases like cancer and Parkinson’s. Find out how you can help us make a difference.
- 122 peer-reviewed papers published in 2024, 63 of which were in high-impact journals
- 15 VAI-SU2C Epigenetics Dream Team clinical trials launched to date
- 10 clinical trials co-funded by VAI & Cure Parkinson's (out of 41 total International Linked Clinical Trials Program trials)
Melissa Hoyer, Ph.D.
Assistant Professor, Department of Neurodegenerative Science
Areas of Expertise
Neurodegeneration, proteomics, neuronal development, cell biology, organelle dynamics, lysophagy, ER-phagy
Biography
Dr. Melissa Hoyer investigates the fundamental cellular processes that support neuronal health with a focus on organelle proteome landscapes. Her leading-edge research has deep implications for understanding the mechanisms that drive protein and organelle degradation, which are defective in neurodegenerative diseases.
Dr. Hoyer earned a B.S. in molecular biology and chemistry from University of Wyoming followed by a Ph.D. in molecular, cellular and developmental biology from University of Colorado (Adviser: Dr. Gia Voeltz). Her dissertation research helped elucidate the relationship between the endoplasmic reticulum and endosome fission, which has significant implications for signaling receptor sorting and degradation in the endocytic pathway. Her graduate work was supported in part by a National Science Foundation Graduate Research Fellowship.
She then joined the lab of Dr. J. Wade Harper at Harvard Medical School as a postdoctoral fellow, where she investigated organelle structure, function and quality control in stem cells as they differentiate to neurons. Her research has revealed powerful new insights into lysosomes and the endoplasmic reticulum, both of which are cellular organelles with links to protein aggregation in neurodegenerative diseases. She was awarded the Jane Coffin Child Fellowship (2019 –2022) and the Fred and Joan Goldberg Fellowship (2022–2024) in recognition of her scholarship.
In 2024, Dr. Hoyer joined Van Andel Institute’s Department of Neurodegenerative Science. Her lab explores the underlying mechanisms that control organelle protein levels in neurons to decipher how organelle proteome landscapes are linked to organelle structure, dynamics and function. Her research aims to better understand the establishment of neurons and maintenance of neuron cell health — both of which are dysregulated in neurodegenerative diseases.
Featured Publications
*Indicates equal contributions
Hoyer MJ, Smith IR, Paoli, JC, Jiang, Y, Paulo JA, Harper JW. 2024. Combinatorial selective ER-phagy remodels the ER during neurogenesis. Nat Cell Biol 26:378–392.
Eapen V*, Swarup S*, Hoyer MJ*, Paulo J, Harper JW. 2021. Quantitative proteomics reveals the selectivity of ubiquitin-binding autophagy receptors in the turnover of damaged lysosomes by lysophagy. eLife 10:e72328
Westrate LM*, Hoyer MJ*, Nash MJ, Voeltz GK. 2020. Vesicular and uncoated Rab1-dependent cargo carriers facilitate ER to Golgi transport. J Cell Sci 133(14).
Hoyer MJ, Chitwood PJ, Ebmeier, CC, Striepen, JF, Qi, RZ, Old WM, Voeltz GK. 2018. A novel class of ER membrane proteins regulates ER-associated endosome fission. Cell 175: 254–265.e14.
Rowland AA*, Chitwood PJ*, Phillips MJ+, Voeltz GK. 2014. ER contact sites define the position and timing of endosome fission. Cell 159:1027–1041.
+ Denotes previous name
Review Articles
Hoyer MJ, Harper, JW. 2023. ER membrane curvature and ubiquitin as drivers of ER-phagy. Dev Cell Spot 58(14):1219–1220.
Hoyer MJ, Swarup S, Harper JW. 2022. Mechanisms controlling selective elimination of damaged lysosomes. Curr Opin Physiol 29:100590
Salvador-Gallego R, Hoyer MJ, Voeltz GK. 2017. SnapShot: Functions of endoplasmic reticulum membrane contact sites. Cell 171:1224–1224.e1
Phillips MJ+, Voeltz GK. 2016. Structure and function of ER membrane contact sites with other organelles. Nat Rev Mol Cell Biol 1–14.
+ Denotes previous name


Katie Colesa
Assistant Research Technician, Department of Neurodegenerative Science

Bryn Couturier
Assistant Research Technician, Department of Neurodegenerative Science

Jamie Durst, B.S.
Senior Administrative Assistant II, Department of Neurodegenerative Science

Kashfia Neherin, Ph.D.
Laboratory Manager, Department of Neurodegenerative Science

2024 Team Outing






